|
|

Painful
Oral Erosions with a Rash
An 86 year-old woman presents to the Emergency
Department with a 4-day history of rash on her back and
legs, as well as painful sores in her mouth. She denies
having fevers or chills and has not experienced any
arthralgias, dyspnea on exertion, or diarrhea. She has been
able to keep up with her oral fluid intake but feels pain
when she swallows. The patient has a past medical history
of chronic atrial fibrillation, hypertension, glaucoma, and
mild dementia. The patient has had no recent change in her
medication regimen and is taking lisinopril, metoprolol,
aspirin, warfarin, timolol, and folic acid.
On physical examination, the patient is afebrile with blood
pressure and heart rate within normal limits. Her oxygen
saturation while breathing room air is 99%, and her
respiratory rate is 12 breaths per minute. The head, neck,
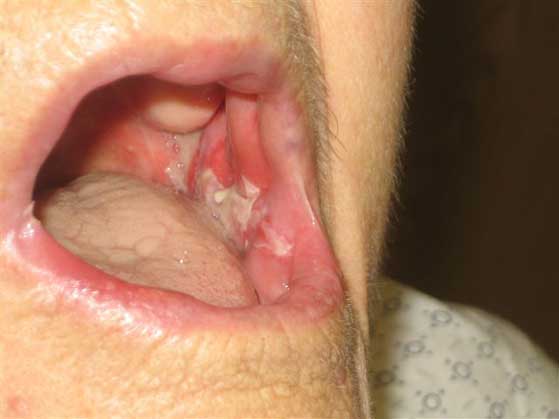
and chest examination is significant for bilateral buccal
mucosal erosions that involve the posterior pharynx,
tongue, and the floor of the mouth (see Images 1-2). The
cardiovascular, pulmonary, and abdominal examinations are
unremarkable. A detailed skin examination (see Image 3)
reveals an erythematous rash with raised plaques mixed in
with patches on the lower back, buttocks, and both thighs.
A few small bullae are also noted. The scalp, face,
conjunctivae, anterior chest, and upper extremities do not
exhibit symptoms.


What is the diagnosis?
Answer
Paraneoplastic
Pemphigus: Further investigation in the
Emergency Department included laboratory findings
that consisted of a complete blood count (CBC) with
differential and a chemistry panel, both within
normal limits. A Dermatology consult was arranged at
the time of the initial visit and a skin biopsy was
performed. Since the patient appeared well and
generally was able to tolerate oral liquid intake,
even though there was a component of odynophagia,
she was discharged to home on oral corticosteroids
(prednisone [1mg/kg every other day]) and a
medicated mouthwash (tetracycline [1.5g] and
nystatin [3 X 106 units] + hydrocodone
[65mg] all mixed into a 500mL elixir of
diphenhydramine [12.5mg/5mL]). As there was no
evidence of bacterial superinfection of any of her
skin lesions, no antibiotics were prescribed.
Followup examinations were arranged with both the
Dermatology clinic and the patient’s primary care
physician.
The skin biopsy results demonstrated prominent
interface change at the dermoepidermal junction,
with intraepidermal and predominantly subepidermal
vesicle and bulla formation. Numerous necrotic
keratinocytes were also seen at all levels of the
epidermis. Superficial perivascular and interstitial
lymphocyte–predominant inflammatory infiltrates
were present, including rare eosinophils. The
histologic features were interpreted as suggestive
of paraneoplastic pemphigus (PNP).
Autoimmune blistering diseases include pemphigus
vulgaris, PNP, bullous pemphigoid, cicatricial
pemphigoid, dermatitis herpetiformis, and linear
immunoglobulin A (IgA) dermatosis. A rare condition,
PNP usually has an onset at age 60 years or older
and is more common in women than men. This disease
is distinct from the classic forms of pemphigus and
is characterized by extensive mucocutaneous erosions
in the presence of a neoplasm, most often leukemia
or lymphoma. Other neoplasms associated with PNP,
both malignant and benign, include Waldenström
macroglobulinemia, sarcomas, thymomas, and Castleman
disease.
Patients with PNP often present with painful oral
mucosal erosions accompanied by a generalized
cutaneous eruption. The earliest and most common
clinical finding in PNP, however, is painful oral
erosions. The erosions can occur anywhere in the
mouth, including the buccal mucosa, labia, gingiva,
and lingual mucosa. The cutaneous eruptions may
initially present with erythema but usually develop
into bullae and erosions and can assume a wide
variety of morphologies, including morbilliform,
urticarial, bullous, papulosquamous, or a rash with
lesions resembling those of erythema multiforme.
Some patients report pruritus or pain over the area
of the cutaneous involvement. In addition to oral
involvement, biopsy-confirmed PNP has also been
reported in the gastrointestinal tract and the
respiratory mucosa. Involvement of the respiratory
mucosa has been increasingly recognized, manifesting
as obstructive lung disease that can progress to
bronchiolitis obliterans and lead to significant
morbidity and mortality.
On histopathologic examination, PNP appears to be a
combination of pemphigus vulgaris and erythema
multiforme. The suprabasilar acantholysis seen in
pemphigus vulgaris is present, as well as basal cell
vacuolation, lymphocytic exocytosis, and
dyskeratotic keratinocytes typical of erythema
multiforme. Interface dermatitis is frequently found
in PNP, both with and without acantholysis.
Exocytosis of inflammatory cells into the epidermis
is common; the amount and the degree of exocytosis
are directly proportional to the degree of
dyskeratosis.
Direct immunofluorescence microscopy of the
patient’s skin shows deposits of IgG and
complement on the surface of the keratinocytes and
other similar immunoreactants in the epidermal
basement membrane zone to varying degrees. Patients
with PNP have IgG autoantibodies against cytoplasmic
proteins that are members of the plakin family (eg,
desmoplakins I and II, bullous pemphigoid antigen 1,
envoplakin, periplakin, and plectin), and against
cell-surface proteins that are members of the
cadherin family (eg, Dsg3). Immunoprecipitation and
immunoblotting are the standard diagnostic tests for
PNP because both of these techniques have
comparatively higher specificities and sensitivities
than indirect immunofluorescence (IDIF) testing.
Unfortunately, neither test is widely available;
however, they can be performed in some research
settings.
Initially, patient care is aimed at treating
superinfection, if present. Standard therapy with
warm compresses, nonadherent wound dressings, and
oral antibiotics is indicated. The administration of
a potent immunosuppressive agent is required to
decrease blistering, but this therapy can often be
ineffective. In general, skin lesions are more
responsive to therapy than mucosal lesions. Other
therapeutic options include plasmapheresis and
immunophoresis. In cases in which a solid neoplasm
is the underlying malignancy leading to the rash,
curative resection should be attempted when
appropriate, but even this may not halt disease
progression.
In general, the prognosis of PNP is poor; however,
when the disease is associated with benign tumors,
the prognosis is somewhat better. The mortality rate
when PNP is associated with malignant tumors is
estimated at 90%. Nearly all patients with the 2
most common associated tumors, non-Hodgkin lymphoma
and chronic lymphocytic lymphoma, have a high
mortality within 2 years of diagnosis. PNP is the
only form of pemphigus that affects the epithelium
of the respiratory mucosa, which manifests
clinically as dyspnea in the setting of normal chest
radiograph findings and can indicate a progression
to bronchiolitis obliterans. The most recent
estimates are that approximately one third of deaths
from PNP are due to pulmonary insufficiency.
References
- Anhalt GJ, Kim
SC, Stanley JR, Korman NJ, Jabs DA, Kory M,
Izumi H, Ratrie H 3rd, Mutasim D, Ariss-Abdo L,
et al. Paraneoplastic pemphigus. An autoimmune
mucocutaneous disease associated with neoplasia.
N Engl J Med. 1990 Dec
20;323(25):1729-35. [MEDLINE 2247105]
- Wakahara M,
Kiyohara T, Kumakiri M, Ueda T, Ishiguro K,
Fujita T, Amagai M, Hashimoto T. Paraneoplastic
pemphigus with widespread mucosal involvement. Acta
Derm Venereol. 2005;85(6):530-2. [MEDLINE
16396806]
- Tilakaratne W,
Dissanayake M. Paraneoplastic pemphigus: a case
report and review of literature. Oral Dis.
2005 Sep;11(5):326-9. [MEDLINE 16120122]
- Bickle K, Roark
TR, Hsu S. Autoimmune bullous dermatoses: a
review. Am Fam Physician. 2002 May
1;65(9):1861-70. Review. [MEDLINE 12018809]
- Kasper DL,
Braunwald E, Fauci A, Hauser S, Longo D, Jameson
JL. Harrison’s Principles of Internal
Medicine. 16th ed. New York, NY: McGraw-Hill
Professional; 2004
- Goldberg LJ,
Nisar N. Pemphigus, Paraneoplastic. Available
at: www.emedicine.com/derm/topic535.htm.
Accessed: March 1, 2007.
|
Link
to further Information on:

http://www.emedicine.com/derm/topic535.htm
http://www.emedicine.com/derm/topic661.htm
|
|







 DISCLAIMER:
This website is designed primarily for use by qualified
physicians and other medical professionals. The
information provided here is for educational and
informational purposes only. It is not guaranteed to be
correct and should NOT be considered as a substitute for
the advice of an appropriately qualified expert. In no way
should the information on this site be considered as
offering advice on patient care decisions or establishment
of a patient-physician relationship.
DISCLAIMER:
This website is designed primarily for use by qualified
physicians and other medical professionals. The
information provided here is for educational and
informational purposes only. It is not guaranteed to be
correct and should NOT be considered as a substitute for
the advice of an appropriately qualified expert. In no way
should the information on this site be considered as
offering advice on patient care decisions or establishment
of a patient-physician relationship.